Chemistry Projects
Buffer Solution
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small or moderate amount of strong acid or base is added to it and thus it is used to prevent changes in the pH of a solution. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications.
Buffer solutions achieve their resistance to pH change because of the presence of an equilibrium between the acid $HA$ and its conjugate base $A^-$.
$$HA \: \rightleftharpoons \: A^- + H^+$$
Working
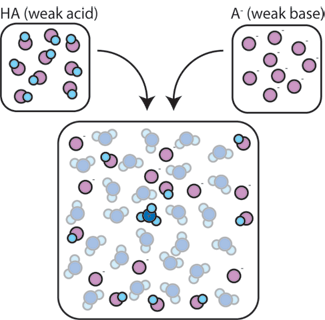
If we mix a weak acid ($HA$) with its conjugate base ($A^-$), both the acid and base components remain present in the solution. This is because they do not undergo any reactions that significantly alter their concentrations. The acid and conjugate base may react with one another, $HA + A^-\: \rightarrow \:A^- + HA$, but when they do so, they simply trade places and the concentrations $[HA]$ and $[A^-]$ do not change. In addition, $HA$ and $A^-$ only rarely react with water. By definition, a weak acid is one that only rarely dissociates in water (that is, only rarely will the acid lose its proton $H^+$ to water). Likewise, since the conjugate base $A^-$ is a weak base, it rarely steals a proton $H^+$ from water.
So, the weak acid and weak base remain in the solution with high concentrations since they only rarely react with the water. However, they are very likely to react with any added strong base or strong acid.
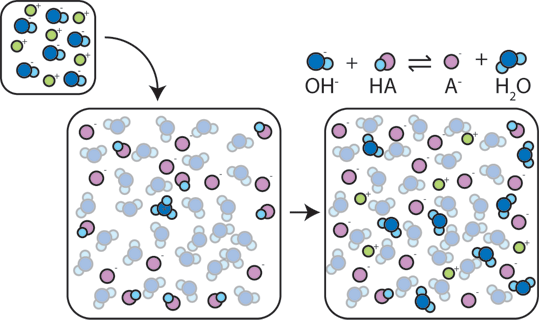
If a strong base is added to a buffer, the weak acid will give up its $H^+$ in order to transform the base ($OH^-$) into water ($H_2O$) and the conjugate base: $HA + OH^- \rightarrow A^- + H_2O$. Since the added $OH^-$ is consumed by this reaction, the pH will change only slightly.
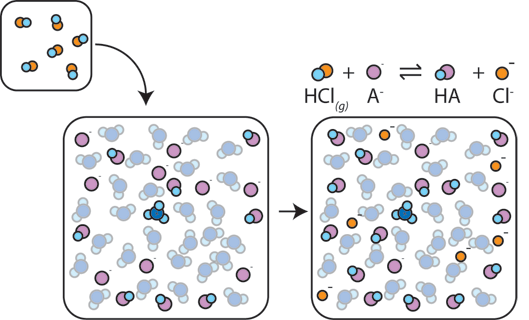
If a strong acid is added to a buffer, the weak base will react with the $H^+$ from the strong acid to form the weak acid $HA$: $H^+ + A^- \rightarrow HA$. The $H^+$ gets absorbed by the $A^-$ instead of reacting with water to form H3O+ ($H^+$), so the pH changes only slightly.
Predicting pH of a Buffer
The pH of a buffer is determined by two factors; 1) The equilibrium constant $K_a$ of the weak acid and 2) the ratio of weak base $[A^-]$ to weak acid $[HA]$ in solution.
1) Different weak acids have different equilibrium constants ($K_a$). $K_a$ tells us what proportion of $HA$ will be dissociated into $H^+$ and $A^-$ in solution. The more $H^+$ ions that are created, the more acidic and lower the pH of the resulting solution.
2) The ratio of $A^-$ to $HA$ in a buffer also affects the pH. If a buffer has more base than acid, more $OH^-$ ions are likely to be present and the pH will rise. If a buffer has more acid than base, more $H^+$ ions are present and the pH will fall. When the concentrations of $A^-$ and $HA$ are equal, the concentration $H^+$ is equal to $K_a$, (or equivalently pH = $pK_a$).
This is formalized in the Henderson-Hasselbalch equation:
$$pH = pK_a + log{\huge(}\frac{base}{acid}{\huge)}$$
Since $pK_a$ is a property of the weak acid we select for our buffer, we can control the pH by manipulating the proportion of weak base ($A^-$) and weak acid ($HA$) in solution.
When $[A^-]/[HA]$ remains close to 1, the pH remains close to $pK_a$. As $[A^-]/[HA]$ goes from 1/10 to 10 the pH changes from $pK_a+1$ to $pK_a-1$. So as long as $[A^-]/[HA]$ is between 1/10 and 10, the pH is within 1 unit of $pK_a$. The addition of a strong acid or base to a buffer changes the ratio $[A^-]/[HA]$. When a buffer absorbs an acid ($H^+$), the acid converts $A^-$ to $HA$ ($A^- + H^+ \rightarrow HA$) and so the ratio $[A^-]/[HA]$ decreases. When a buffer absorbs base ($OH^-$), the base converts $HA$ to $A^-$ ($HA + OH^- \rightarrow A^-$) and so the ratio $[A^-]/[HA]$ increases. As long as the added acid or base doesn't cause $[A^-]/[HA]$ to go outside the range 1/10..10, the pH will remain within 1 unit of $pK_a$, i.e. the pH won't change by very much and the solution is therefore buffered.